Cancer is a general term used for a group of disorders, which manifest in most cases as an abnormal mass/lump/swelling formed due to abnormal cells that multiply uncontrollably, and which have the potential to invade other tissues, both local and distant.
Our body is a well-organized collection of trillions of small structures called cells. Cells are the structural and functional unit of living tissue. They are to a living tissue, what an atom is to an element or a molecule to a compound. It is estimated that, in an adult human body, there may be anywhere between 50 to 75 trillion cells. But, how much is a trillion? A thousand billion makes a trillion; an unimaginably large number. To understand the enormity of this figure, let us suppose you start counting each one of the cells in your body at a rapid rate of 50 to 75 cells per second; how long do you think you will take to finish counting? Well, without taking any sort of break, including for food or sleep, it would take you 31,688 long years! Clearly, our body is an incredibly complex biological machine made of these trillions of parts.
In a normal human being, each one of these trillions of cells responds and functions according to the needs of the body. A group of cells form a tissue or an organ. Depending on the tissue or the organ, cells are of different types. Normally, these cells grow, divide and form new cells, or die, as per the physiological needs of the body. In other words, in a healthy person, the body controls the growth and multiplication of the cells according to its needs, and the cells strictly comply with these rules. In a normal adult, about 3 million red blood cells die every second while in the same time the remaining tissues of the body lose about one million cells! These are constantly replaced by formation of new cells. The new cells formed are almost exact copies of the ones that have died, and they take over the function of these lost cells. Unusually, a new abnormal cell is created, which fails to obey the strict control on cell growth, multiplication and function. This happens due to some permanent alteration in the cell’s genetic material - the DNA, either spontaneously or due to the influence of chemicals, radiation, etc. This process is known as a mutation.
Considering the huge production of new cells in the body every second, it is a marvel that this event is very rare. This is because there are highly efficient ‘proofreading’ mechanisms, which check for errors in the formation of the new DNA and remove them.(Here, it is important to know that mutations are not always bad. In-fact, we owe the evolution of our entire biological world from a single celled organism into the biodiversity around us, including ourselves, largely to mutations. Mutation is basically a change in the DNA which escapes the ‘proofreading’ system and therefore, becomes established in the genetic code of that cell and can hence then be passed on to its daughter cells, when it divides and multiplies. Some of these mutations can give rise to the formation of a tumor cell.). This abnormal mutant cell, instead of responding to the needs of the body, starts to grow and multiply in an uncontrolled manner. This gives rise to the formation of more abnormal cells, and since these are copies of the original mutant cell, they behave exactly like the parent cell, i.e. they too, in turn, grow and multiply uncontrollably; this cycle keeps continuing with every new generation of abnormal cells. This event is thus like a chain reaction that eventually leads to the formation of a lump or a swelling, which is called a tumor or tumour.
These mutant cells, and the tumor tissue formed by them, are also abnormal in their structure, architecture and function. They grow more rapidly and uncontrollably, as compared to the normal cells and tissues around. Thus they affect the body in several ways. They take away nourishment from the normal tissues and grow at their expense. Tumor tissue is not normal tissue and therefore, it does not serve the function of the tissue it was supposed to be. And since they grow uncontrollably, they invade the space occupied by the normal tissues and replace them, thus compromising their function. However, all tumors are not cancers. An important behaviour, which is the hallmark of cancer, is the ability to spread from the organ or tissue of origin, to other parts of the body. This is the most dangerous characteristic of cancer. Cancer is thus a general term used for a group of disorders, which manifest in most cases as an abnormal mass/lump/swelling formed due to abnormal cells that multiply uncontrollably, and which have the potential to invade other tissues, both local and distant.
Yes, most certainly. Bone is a living tissue. Therefore, bone cancer is possible and is well known.
In a simple language, bone cancer is cancer affecting the bones. However, bone cancer is of two types:
- Primary bone cancer: This is a form of bone cancer where the cancer begins in the tissue of the bone itself. They can be of various types de1pending on the nature of the tumor tissue. The common types of primary bone cancers are osteosarcoma, chondrosarcoma and Ewing’s sarcoma. Primary bone cancer is quite rare and account for less than 1% of all cancers. In this section, we will be discussing primary bone cancers only.
- Secondary bone cancer: This is also called as bone metastasis or skeletal metastasis. This is a form of bone cancer, where the cancer has spread to the bone from a primary cancer arising in some other organ or tissue. The most common cancers which spread to the bone are from the breast, prostate, lungs, kidneys and thyroid. Secondary bone cancer is far more common than primary bone cancer. To know more about secondary bone cancer, To know more about secondary bone cancer, read the page on bone metastasis.
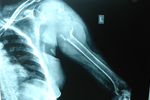
Sarcoma is a class of cancer arising from connective tissue, which includes bone, muscle, fat tissue, etc. The other major classes of cancer are carcinoma, lymphoma, leukaemia, etc. Almost all primary bone cancers are sarcomas.
 Sarcoma can affect any bone in the human body. However, some regions are more commonly affected than the rest. The most commonly affected are the bones around the knee, hip and shoulder joints and the pelvic bones.
Sarcoma can affect any bone in the human body. However, some regions are more commonly affected than the rest. The most commonly affected are the bones around the knee, hip and shoulder joints and the pelvic bones.
The most common symptoms are pain and/or swelling. Some patients may present with a pathological fracture. A bone tumor in the region of a joint often causes restriction in the range of movement and function of the joint, either due to a mechanical obstruction caused by the swelling or due to the pain. When a sarcoma affects a vertebra, it can cause pain in the back, and if it compresses the spinal cord, it can cause weakness and numbness in the distal part of the body.
The most common symptoms are pain and/or swelling in the affected area. The pain initially may be felt only on activity, i.e. on using the affected part of the body, e.g. walking may cause/aggravate pain in the lower limbs and lifting or throwing may trigger pain in the upper limbs. Soon, the pain may become more persistent and remain present even at rest, and eventually may become continuous and affect sleep. In some patients pain may be felt at rest, right from the beginning. The pain may be relieved initially, with painkillers. However, the pain often returns within a short time after stopping the medication. A swelling may become noticeable in the painful area at the same time as the pain, or sometime later. Often, the swelling may appear before any pain or discomfort is felt. Sometimes, especially in children, the disease can present as a limp while walking, or difficulty in using the upper limb for routine activity. When present around a joint, a bone tumor can cause restriction in the range-of-movement and function of that joint, either due to a mechanical obstruction caused by the swelling or due to the pain.
Rarely, the first sign of trouble may be fracture of the affected bone. This is called a pathological fracture or pathologic fracture. The hallmark of this kind of fracture is that, it occurs without a significant cause. It may occur while standing up or walking, or with a trivial fall, or even with a sudden jolt experienced while travelling in a vehicle. However, it is important to know that pathological fracture can be caused by many conditions, other than bone tumors. One of the commonest causes of pathological fracture is osteoporosis.
Sometimes, the disease may affect a vertebra (one of the series of bones that form the backbone/spine, which houses the spinal cord in a hollow canal within) and may present with pain in the back. If the tumor grows out of the confines of the bone and exerts pressure on the spinal cord, it may cause weakness and/or numbness in the distal part of the body, a problem which is noticed earliest, and most often, in the legs. This may sometimes also result in the loss of voluntary control over the passing of urine and stools.
Primary bone cancer (sarcoma) can affect anybody. There is no real way to identify persons at risk. It can occur at any age. However, some bone cancers affect particular age groups more frequently than others.
Primary bone cancer (sarcoma) can affect anybody. There is no real way to identify persons at risk. It can occur at any age. However, some bone cancers affect particular age groups more frequently than others. For example, primary bone cancers like Osteosarcoma and Ewing’s Sarcoma are most common in the first two decades of life, whereas Chondrosarcoma is unusual before the age of 25 years. There are some rare situations, which have been associated with an increased risk of bone sarcomas, e.g. in those who have received radiation to the bone for the treatment of some benign bone tumor (like Giant Cell Tumor), those with Paget’s disease, bone infarctions, etc.
This may seem a silly question as most people are aware what cancer means. What is more important to know is, it is treatable, and that, earlier the condition is detected and treated, better is the outcome. This is just to reinforce that information. Yes, as with any cancer, bone sarcoma is also dangerous and can be life threatening if not detected in time and treated appropriately.
If detected early and treated appropriately, cure is certainly possible in primary bone cancer. However, it is important to establish correctly, the diagnosis of sarcoma, the type of sarcoma, and the stage of the disease.
There is no known cause for bone sarcoma and consequently, there is no general preventive precaution that can be taken or advised. It can affect anybody.
Unlike some cancers like lung cancer (which has been related to smoking), and mouth cancer (which has been related to the use of tobacco), etc., where some significant causative influence is known, there is no known cause for bone sarcoma and consequently, there is no general preventive precaution that can be taken or advised. It can affect anybody. Therefore, the emphasis is on early detection, correct diagnosis and right treatment.
One specific precaution that can be advised is to avoid radiotherapy of any benign bone tumor (especially GCT of bone), unless all other options have been exhausted, as it is associated with a risk of formation of bone sarcoma in the treated area.
There are certain rare syndromes and familial genetic disorders, which have been associated with an increased risk of bone sarcomas. They are discussed in more detail in a subsequent section.
In such an event, the most important thing that you must do is, speak to your doctor and consult a bone tumor specialist. However, in the meanwhile, there are several things that you must NOT do. They are as follows:
- Do not massage the swelling under any circumstance. This is because, if it is a malignant tumor, massaging could cause some of the tumor cells to be released into the blood circulation or the lymphatic system, through which they could travel and lodge in some distant organ or tissue and form metastasis. Besides, massaging will not offer relief in the presence of a tumor; it could actually aggravate or trigger the pain. It can also cause a pathological fracture.
- For the same reason as above, do not apply any sort of compression bandage over the swelling. Also, avoid touching and examining the region frequently.
- Do not attempt or allow, any sort of chiropractic manipulation of the affected region. This could be dangerous in the presence of a bone tumor. Besides the risk of releasing tumor cells into the blood and lymphatic circulation, it can also cause a pathological fracture.
- A bone with tumor generally does not have the strength of a normal bone as most tumors tend to weaken them; which means such a bone is at a risk of fracture following trivial events like lifting a heavy object, a fall at home, walking, etc. (pathological fracture). Hence all precautions must be taken to avoid the possibility of such a fracture.
It is best to follow the following guidelines till your doctor examines you and decides what level and what kind of activity is safe for you:
-
Avoid unnecessary use of any limb which is painful or has a swelling.
- Avoid any activity which triggers or aggravates the pain.
- Do not attempt to lift heavy objects.
- If the tumor is in the lower extremity, avoid putting weight on that limb while walking or standing. Use a ‘walker frame’ if necessary.
- Avoid falling, at all costs. Any kind of local injury, in the presence of a bone tumor, carries a significant risk of causing a pathological fracture. This often happens where and when you least expect it – ‘at home’. Most often, this happens in the toilet or the bathroom due to a slip on the polished floor tiles, which may be wet and therefore, slippery. These are also situations when you are generally alone. Seek assistance while ‘going to’ and ‘coming from’ these places. Beware of wet floors. A pathological fracture, besides causing intense pain and serious disability, also complicates the treatment of the disease.
ICertainlynot. This is not a contagious disease or any sort of infection. There is no health risk to any member of the family whatsoever.
It is very unusual for sarcomas to run in families, although such occurrences are not totally unknown. Most cases of sarcoma, in general, have no known cause. The best explanation available now is that, in most cases, it’s merely a matter of chance (i.e. they are sporadic or of spontaneous origin). In such a situation, the risk to any member of the family (be it, a sibling, offspring, parent or any other blood relative), is for all practical purposes, similar to that of an unrelated person. However, there are certain very rare genetic disorders that run in some families, which have been associated with an increased risk of developing malignant tumors, including sarcomas, e.g. Li-Fraumeni syndrome.
It is very unusual for sarcomas to run in families, although such occurrences are not totally unknown. Most cases of sarcoma, in general, have no known cause. The best explanation available now is that, in most cases, it’s merely a matter of chance (i.e. they are sporadic or of spontaneous origin). In such a situation, the risk to any member of the family (be it, a sibling, offspring, parent or any other blood relative), is for all practical purposes, similar to that of an unrelated person. However, there are certain rare genetic disorders that run in families, which have been associated with an increased risk of developing malignant tumors, including sarcomas, e.g. Li-Fraumeni syndrome. On the other hand, there are certain rare benign bone tumor conditions, which have known genetic cause, some of which may also be part of a syndrome, e.g. McCune-Albright syndrome, Hereditary Multiple Exostosis (HME), etc. There also are other rare benign bone tumor conditions where the exact cause is not known, but a genetic cause is suspected, e.g. Multiple Enchondromatosis (Ollier’s disease), Maffucci syndrome, etc. An interesting feature of these benign bone tumor conditions is that they are associated with the formation of bone lesions in multiple locations in the skeletal system. Despite having a known or a suspected genetic cause, a majority of these disorders are sporadic in origin (with exceptions like HME), i.e. they are not inherited and therefore, do not indicate an increased risk of a similar condition to the other blood-related family members. A rare example of an inherited bone tumor disorder is HME. This is an autosomal dominant disorder which causes the formation of multiple osteocartilaginous exostoses, which are benign outgrowths of bone capped by cartilage. Due to the occurrence of multiple tumors in this condition, one or more of which may undergo a malignant transformation, it poses a significant risk to the patient, of developing a sarcoma. Similar risk of developing a sarcoma (for a similar reason) exists in some of the other benign bone tumor conditions mentioned earlier, e.g. Multiple Enchondromatosis (Ollier’s disease), Maffucci syndrome, etc.
Primary bone cancer is most commonly detected when a patient reports to his/her doctor with some unusual pain or/and swelling in the body. Uncommonly, some patients may present with a pathological fracture. Some patients with disease involving the spine, may present with back-pain and sometimes also with signs of spinal cord compression. The simplest and most important investigation to detect primary bone cancer is a regular plain X-ray. Most cases of bone sarcoma can be detected and identified on an X-ray. The other investigations of value are MRI, Bone scan, CT scan and PET scan.
Primary bone cancer is most commonly detected when a patient reports to his/her doctor with some unusual pain or/and swelling in the body. Uncommonly, some patients may present with a pathological fracture. Some patients with disease involving the spine may present with back-pain, and sometimes, also with signs of spinal cord compression. A detailed history and clinical examination helps in localizing the problem and also in deciding the further line of investigation. The simplest and most important investigation to detect primary bone cancer is a regular plain X-ray. Most cases of bone sarcoma can be detected and identified on an X-ray. The other investigations of value are MRI, Bone scan, CT scan and PET scan. However, all these investigations are generally done after viewing the X-ray. The need for these other investigations will be decided by the doctor treating you.
The foremost thing that you must do is consult your doctor, when you notice any unexplained swelling on your body (even if it is painless and does not cause any discomfort), or have some unexplained pain that refuses to go away or is increasing in duration and/or intensity. However, there is no reason to panic or be alarmed. In most cases, this is usually due to some routine cause, which can be easily explained and treated. Nonetheless, it is always wise to be aware and vigilant. It is important to remember that, earlier a cancer is detected and treated, better is the benefit of treatment, which in turn gives the best opportunity to overcome the disease.
Merely finding a suspicious problem in the bone on a radiological investigation is not necessarily a diagnosis of bone cancer. It is always important to establish the diagnosis by doing a biopsy.
Merely finding a suspicious problem in the bone on a radiological investigation is not a final diagnosis of bone cancer. Although, in a large number of cases, a plain X-ray does give definite clues about the nature of the lesion in the bone, including the likely diagnosis, it is always important to establish the diagnosis by doing a histological study of the tumor tissue (except in some well-known well-identified non-aggressive benign bone conditions). This study is done by a pathologist on a sample of the tumor tissue which is obtained through a minor surgical procedure called a Biopsy. Bone cancer as we already know, could be either primary or secondary. Moreover, there are different types of primary bone cancer and different sources of secondary bone cancer. It is very important to know the exact diagnosis of the cancer affecting the bone, as the treatment plan is different for different types of cancer. It is also important to know that non-cancerous (benign) bone tumors are much more common than cancerous (malignant) bone tumors. Furthermore, there are certain non-tumorous conditions, which can behave and look like a bone tumor, both clinically and on radiological investigations. One such common condition which can mimic a bone tumor is bone infection (more appropriately called as Osteomyelitis). A biopsy can easily settle these issues.
Biopsy is a minor surgical procedure in which a small piece of the tumor tissue is sampled and sent for evaluation to a pathologist, who examines it under a microscope to establish its exact histological identity/name (diagnosis). An improperly done biopsy can affect the future treatment of the disease, especially if it turns out to be a sarcoma. Leaving behind the biopsy scar and track, at the time of definitive surgery for the removal of a sarcoma, increases the risk of local recurrence of the disease significantly. It is therefore, very important that the biopsy is conducted in a well-planned and careful manner, ensuring the least possible contamination of the normal tissues forming the biopsy track, with the tumor tissue/cells. It is also equally important that the biopsy site is placed in an ideal location, which would allow the scar and track of the biopsy to be easily included for excision, along with the tumor, during the definitive surgery. Biopsy is therefore, best done by a surgeon who is experienced in bone tumor treatment.

Biopsy is a minor surgical procedure in which a small piece of the tumor tissue is sampled and sent to the laboratory for evaluation by a pathologist. The pathologist, after processing the tissue sample, examines thin slices of it under a microscope to identify whether it is cancerous tissue and if so, what type of cancer it is, i.e. its exact histological identity/name (diagnosis). The pathologist can find out whether it is primary bone cancer or secondary bone cancer. In cases of primary malignant tumor of bone (sarcoma), the pathologist, other than identifying the exact type of sarcoma, can also opine on the grade of the tumor (whenever relevant), which is of value in ‘staging’ the disease. In case of secondary bone cancer, the pathologist can often identify the source of the cancer (i.e. in which distant organ, the cancer originated from), which helps in localizing the primary source cancer, and thus in initiating effective treatment.
An improperly done biopsy can affect the future treatment of the disease, especially if it turns out to be a primary malignant bone tumor. It is a rule in musculoskeletal tumor surgery to remove the scar of biopsy along with the biopsy track (with a margin of surrounding normal tissue) en masse with the tumor, during the definitive surgery for a sarcoma, as it is considered to be a contaminated zone (an area which has come in contact with tumor tissue and therefore, could now be seeded with tumor cells). It is well documented that, leaving behind the biopsy scar and track, at the time of the definitive surgery for the removal of a sarcoma, increases the risk of local recurrence of the disease significantly. This makes it essential that the biopsy is conducted in a well-planned and careful manner, ensuring the least possible contamination of the normal tissues forming the biopsy track (tissues through which the biopsy track cuts through) with the tumor tissue/cells. It is vital that the biopsy incision is placed in an ideal location, which would allow the biopsy scar and track to be easily included for removal, along with the tumor, during the definitive surgery. An MRI is very helpful in planning the optimal location and approach of the biopsy. An incorrect biopsy location/approach, or an improperly done biopsy, may force the surgeon to remove important tissues (which could otherwise have been saved) along with the tumor, just to include the biopsy scar and track in the resection (to achieve en bloc excision with a wide margin). This could either result in a loss of useful function, or may necessitate complex reconstruction procedures, involving plastic surgeons, vascular surgeons, etc., to attempt to restore useful function and/or save the limb. It is well documented that, in some unfortunate situations, a poorly done biopsy procedure may leave no treatment option except amputation, in an otherwise perfectly salvageable extremity. Biopsy is therefore, best done by a surgeon with experience in bone tumor treatment.
Several biopsy techniques are available, including fine needle aspiration biopsy, core needle biopsy, incisional biopsy, excisional biopsy, etc. Biopsy for bone tumors is generally done as a small open surgery (Open Biopsy), or with the help of special biopsy needles (Core Needle Biopsy). Between the two, Core Needle Biopsy is generally the preferred method for doing a biopsy as it is a simple, reliable, minimally invasive, safe and quick procedure. The correct method for your situation is best decided by your doctor.
‘Grade’ of the tumor is a histological calculation of the aggressiveness of a malignant tumor (more specifically, its ability to metastasize, i.e. the ability to spread to other regions of the body). Grading of the tumor is done by the pathologist based on the histological observations made on the tumor tissue. This information tells the clinician about the extent of risk, the patient has, of developing metastasis. There are several different systems for grading a malignant tumor.
‘Grade’ of the tumor is a histological calculation of the aggressiveness of a malignant tumor (more specifically, its ability to metastasize, i.e. the ability to spread to other regions of the body). Using formal criteria, the pathologist, after observing thin slices of the disease tissue under a microscope, allots a particular grade to that tumor. This information tells the clinician about the extent of risk, the patient has, for developing metastasis. The grade for a particular tumor may differ according to what system of grading is followed by the pathologist. Musculoskeletal Tumor Society (MSTS) system classifies malignant bone tumors as either low-grade (G1) or high-grade (G2). American Joint Committee on Cancer (AJCC) System (also called the TNM System) classifies these tumors into four grades - G1 (well differentiated), G2 (moderately differentiated), G3 (poorly differentiated) and G4 (undifferentiated). G1 and G2 tumors of AJCC system is considered equivalent to the G1 tumors of MSTS system (low-grade tumors), while G3 and G4 tumors of AJCC system are considered equivalent to the G2 tumors of MSTS system (high-grade tumors). Not all tumors are graded, as some tumors invariably show consistent behavior, e.g. Ewing’s Sarcoma (Ewing’s Sarcoma is always a high grade disease. There is nothing like a low grade Ewing’s Sarcoma. Therefore, grading of Ewing’s Sarcoma is of no practical value, as just the diagnosis of Ewing’s Sarcoma implies a high grade disease.)
‘‘Core Needle Biopsy’ in skilled hands, offers significant advantages over ‘Open Biopsy’ and therefore, it is the preferred method for obtaining tumor tissue samples.

The goal of a biopsy is to obtain a sample of tumor tissue so that it can be studied by the pathologist to identify the exact nature of the disease and establish its identity, as treatment of any tumor depends on its diagnosis. Generally, it is possible to obtain a larger quantity of tissue sample (which makes it easier for the pathologist to process and study the material) through an Open Biopsy. However, the material obtained by Core Needle Biopsy, in most cases, is adequate for a skilled pathologist. In many other ways, the advantages and benefits offered by a CNB are superior to that of an OB. As mentioned earlier, CNB is a simple, reliable, minimally-invasive, safe and quick procedure.
In the following table, a comparison is made between OB and CNB:
Sr. No. |
Points of comparison |
Open Biopsy |
Core Needle Biopsy |
01 |
Nature of procedure |
Invasive |
Minimally-invasive |
02 |
Size of incision |
Depends on depth of tumor. Deeper the disease, larger the incision required to reach it. Ranges from about 3 - 4 cm. up to several cm. |
Does not depend on depth of tumor. Size of incision always remains the same; about 5 mm. In-fact, therefore, deeper the tumor, more is the advantage of needle biopsy. |
03 |
Tumors in complex locations like spine, pelvis, sacrum, etc. |
As these lesions are deep and in difficult regions, open biopsy is highly unsuitable. |
Most effective procedure for these lesions. Can be done under image guidance like CT scan, etc. |
04 |
Quantity of tissue obtained |
Substantial amount of tissue can be obtained. |
Less tissue obtained – however, adequate for a skilled pathologist. |
05 |
Drain tube placement following the procedure |
May be needed; to drain the collected blood (haematoma) |
Never required |
06 |
Blood loss |
May be significant |
Generally, limited |
07 |
Ease of procedure |
Easy |
Requires skill |
08 |
Reliability |
Excellent |
Excellent in skilled hands |
09 |
Safety |
Excellent |
Excellent |
10 |
Possibility of fracture following the procedure |
Likely; especially if the biopsy is from the diaphyseal region (shaft area) of a long bone |
Very unlikely |
11 |
Speed |
Takes much longer time than needle biopsy |
Few minutes |
12 |
Anaesthesia |
May need general or regional anaesthesia |
Most often, done under local anaesthesia |
13 |
Recovery from procedure |
May take a few hours to a day |
Almost immediate |
14 |
Post-procedure pain |
May be significant |
Usually tolerable, and abates quickly. |
15 |
Hospital stay |
May need a few hours or a day’s stay in the hospital |
Usually an OPD based procedure. Does not generally need hospital admission. |
16 |
Local tissue contamination |
Significant |
Very limited |
17 |
At final surgery |
More skin and normal tissue is lost while removing the biopsy scar and track. |
Minimal loss of skin and normal tissue as the biopsy scar and track is small. |
18 |
Cost of procedure |
Much more as compared to Core Needle Biopsy |
Very reasonable |
It is quite obvious from the above comparison that Core Needle Biopsy in skilled hands, offers significant advantages over Open Biopsy and therefore, it is the preferred method for obtaining tumor tissue samples. Nevertheless, there may be some situations where an open biopsy procedure could be the more suitable option.
Once a diagnosis of bone cancer (sarcoma) is made, the next important step is to ‘Stage’ the cancer.
In simple words, staging is a system to determine the extent of cancer in the patient and the level of risk posed to the patient due to it. Stage of the cancer at diagnosis, is the best predictor of survival and is a powerful guide to the optimal treatment of the disease. One of the most important pieces of information derived from staging investigations is whether the disease is localized or metastatic. The importance of staging is that, treatment strategies can be specifically planned to the patient’s situation, to get the best outcome.
In simple words, staging is a system to determine the extent of cancer in the patient and the level of risk posed to the patient due to it. Stage of the cancer at diagnosis, is the best predictor of survival and is a powerful guide to the optimal treatment of the disease. In cancer management, after a diagnosis of cancer is made, a set of tests are carried out to stage the cancer. Staging may involve several investigations like histopathology, MRI, CT scan, Bone scan, PET scan, etc. The exact set of investigations varies from case to case and will be decided by your treating doctor. Staging criteria may differ slightly for different tumors. One of the most important pieces of information derived from staging investigations is whether the disease is localized or metastatic. It is possible that the patient may have the disease in other parts of the body, but is not aware of it as they are small in size and therefore, are not causing any problem at this moment. Staging investigations help in locating and identifying these metastatic foci of disease early. This helps in choosing the most useful and effective treatment approach to such a patient, giving due consideration to the presence of any metastasis and its treatment. The importance of staging is that, treatment strategies can be specifically planned to the patient’s situation, based on the stage of the disease, to get the best outcome. For bone sarcomas, there are two major staging systems. One is the MSTS (Musculoskeletal Tumor Society) staging system, which classifies these conditions into three stages, with stage ‘I’ being the lowest, offering the best prognosis to the patient and stage ‘III’ representing the highest, indicating metastatic disease and consequent poor prognosis. The other staging system is the AJCC (American Joint Committee on Cancer) staging system (also called the TNM System) which classifies these diseases into four stages with stage ‘I’ being the lowest, offering the best prospect for recovery and stage ‘IV’ denoting the highest, indicating metastatic disease, suggesting the least chance of a least favourable outcome.
‘Grade’ of the tumor tells us how aggressive the disease is; especially about its tendency to metastasize (spread to other regions of the body). However, it does not tell us anything about the extent of its presence in the patient and the actual threat it poses to the patient, at any given time. ‘Staging’, on the other hand, is a system that helps in understanding the extent of cancer in the patient and the level of risk to the patient due to it. ‘Stage of the cancer at diagnosis’ is the best predictor of survival and is a powerful guide to the optimal treatment of the disease.
As described earlier, ‘Grade’ of the tumor is a value allotted to the tumor by a pathologist based on its appearance under the microscope (sometimes, in certain borderline cases, clinical and radiological information may also be used while allotting the grade to the disease). It tells us about the nature of the disease, especially about its tendency to metastasize. However it does not tell us anything about the extent of its presence in the patient and the actual threat it poses to the patient, at any given time. ‘Grade’ of the disease is just one of the criteria used in the staging of cancer. Staging systems take into account several other details to designate the ‘Stage’ of the disease.
For example, let us consider the situation of two patients with bone tumor; ‘Patient A’ and ‘Patient B’. A biopsy revealed that both have Chondrosarcoma. The pathologist further added that the tumor was ‘Low Grade’ in both patients. In conclusion, both patients have identical disease, i.e. Low Grade Chondrosarcoma; in which case it is logical to assume that both ‘Patient A’ and ‘Patient B’ will receive the same treatment. However, that is not correct. We still do not have the complete information necessary for formulating the right treatment plan. We know that cancer can spread to other organs and tissues. It is for this reason, staging investigations are done. Staging investigations revealed that ‘Patient A’ has no metastasis (i.e. he has localized disease only) whereas ‘Patient B’ has metastases in the lungs; which means ‘Patient A’ has ‘Stage I’ disease while ‘Patient B’ has ‘Stage IV’ disease. Clearly, the same treatment strategy will not work for both patients as the extent of disease in both patients is different. So, despite having the same disease with identical Grades, ‘Patient B’ with ‘Stage IV’ disease is at a higher risk as compared to ‘Patient A’ with ‘Stage I’ disease, and therefore needs a different treatment approach. This is essentially the difference between ‘Grade’ and ‘Stage’ of the disease. Thus, staging is a system that helps in understanding the extent of the cancer in the patient and the level of risk to the patient due to it. Stage of the cancer at diagnosis, is the best predictor of survival and is a powerful guide to the optimal treatment of the disease.
At this point it is important to have a clear idea that ‘grading’ and ‘staging’ is meant for primary malignant tumors only (As already described in the section on bone metastases, a metastatic bone disease is considered as a part of the cancer of the tissue or organ from where it had first started, and in effect, represents stage ‘IV” of that cancer.).
Treatment strategies are different for patients in different stages of the disease, including those with localized cancer and those who have metastatic cancer. By considering various criteria, staging not only helps in identifying patients with metastatic disease, but it also helps in identifying people with non-metastatic disease who have a higher risk than some others with localized disease. Knowing that a patient has metastatic sarcoma makes it possible to employ a more aggressive treatment plan to deal with the metastases along with the primary tumor. The stage of the disease at diagnosis is one of the important details, which help in predicting the likelihood of recovering from the condition.
The need for staging is obvious once you understand the behaviour of malignant tumors. Malignant tumors are those tumors which have the ability to spread locally (invasion), and to distant organs and tissues (metastasis). Treatment strategies are different for patients in different Stages of the disease, including those with localized cancer (non-metastatic cancer – disease, which has not spread to distant locations) and those having metastatic disease (cancer which has spread to distant organs/tissues). By considering various criteria, staging not only helps in identifying patients with metastatic disease, but also helps in identifying people with non-metastatic disease who are at a higher risk than some others with localized disease (e.g. due to a higher grade of tumor, larger size of the disease, etc.). AJCC ‘Stage I’, ‘Stage II’ and ‘Stage III’ disease, all represent localized sarcoma, but with increasing levels of risk to the patient due to higher grade of disease, larger size of disease, etc. A patient with localized disease, but with a higher Stage, is at a higher risk of relapse of disease as compared to one with a lower Stage, and therefore, requires more radical or aggressive treatment. Knowing that a patient has metastatic sarcoma makes it possible to employ a more aggressive treatment plan to deal with the metastases along with the primary tumor. If staging investigations are not done, metastasis (if any) may remain unknown for a long time, until it becomes large and causes symptoms. A potentially treatable metastasis may thus become untreatable, if not detected in time and treated appropriately. Sometimes, in patients with extensive disease, staging may help avoid unnecessary unhelpful treatment. The ‘Stage of the disease at diagnosis’ is one of the important details, which helps in predicting the likelihood of recovering from the condition. Generally, lower the Stage of the disease, better is the prognosis; higher the Stage, worse is the likely outcome.
Treatment for a bone sarcoma is started after the diagnosis is established and the staging investigations are completed. If treatment for a malignant tumor is started without staging the disease, it may result in improper therapy as vital information regarding the extent of the disease in the patient would not have been considered in the planning of that treatment.
Treatment for a bone sarcoma is started after the diagnosis is established and the staging investigations are completed. Armed with the information regarding the nature of the disease, its location and size, the extent of its presence in the patient, the general health of the patient etc. a treatment strategy is planned to give maximum benefit to the patient in terms of recovering from the disease and preservation of useful function.
If treatment for a malignant tumor is started without staging the disease, it may result in improper therapy as vital information regarding the extent of the disease in the patient would not have been considered in the planning of that treatment. It is important that staging is done before beginning the treatment because treatment can lower the stage of the disease. Let us, for example, consider the situation of a patient with High Grade Osteosarcoma of the femur bone, who also has lung metastasis, i.e. he has ‘Stage IV’ disease. By doing the staging before starting the treatment, the most appropriate treatment strategy for this patient can be employed, which also includes the treatment of the metastasis. But if the treatment is started before staging the disease, the initial treatment with neoadjuvant chemotherapy would shrink the primary tumor and the tumor metastases. In many cases it can cause the metastasis to disappear. If this patient is staged now, no metastasis will be found, and one would erroneously consider him to be at a lower stage (e.g. ‘Stage II’), which would advocate the use of a different, less aggressive, treatment strategy than what was actually needed for this patient. Thus, staging the patient after starting the treatment could completely change the disease picture and might result in sub-optimal treatment. It is therefore, important to remember that the real value of staging is only when it is at diagnosis, and not after starting of treatment.
The most important treatment modalities for bone sarcoma are Chemotherapy, Surgery and Radiotherapy. Based on the individual case, either one or two or all the three modalities may be employed in the treatment of bone sarcoma.
The exact treatment strategy for a patient with bone sarcoma depends on multiple factors. The most important thing to know is the exact diagnosis of the cancer (i.e. whether it is an Osteosarcoma, Ewing’s sarcoma, Chondrosarcoma, etc.), as the treatment protocol is different for the different types of sarcoma, as they respond differently to the various modalities of treatment. Another important factor is the Stage of the disease, especially, whether the sarcoma is localized or is metastatic. Depending on the diagnosis and the Stage of the disease, the treatment of an individual patient is decided. The most important treatment modalities for bone sarcoma are Chemotherapy, Surgery and Radiotherapy. Based on the individual case, either one or two or all the three modalities may be employed in the treatment of bone sarcoma.
In any cancer treatment, the first thing to be decided, is the ‘Goal’ of the treatment; i.e. whether the goal is to achieve ‘Cure’ or ‘Palliation’. This depends on various factors like, the extent of the disease, the Stage of the cancer, the general health of the patient, etc. As is evident to all, the goal of ‘Cure’ implies that the treatment is directed at complete eradication of the disease from the patient and ensuring the lowest possible risk of relapse of the cancer. On the other hand, ‘Palliation’ implies partial treatment of the condition, the goal being, to control the disease (not cure), so as to ease the symptoms and discomfort caused by it, or, to slow the progression of the disease, so as to avoid or delay the adverse complications due to it; thus offering the patient better survival and better quality of life.
When the intention of care is ‘Palliative’, although surgery certainly has an important role, often the treatment may be limited to just chemotherapy, or in some cases, chemotherapy with radiotherapy. The decision regarding the treatment plan is taken by your treating doctor based on all the available information.
Chemotherapy is one of the foundations of modern bone sarcoma treatment and has revolutionized the management of chemo-sensitive tumors like Osteosarcoma, Ewing’s sarcoma, etc.
Chemotherapy is a form of cancer treatment involving the use of powerful drugs, which are toxic to the cells of living tissue. However, they are more toxic to cancer cells than normal cells. Hence, in the doses which are given to the patient, they kill cancer cells in much larger numbers as compared to normal cells. Nevertheless, chemotherapy does cause significant side effects like hair loss, nausea, vomiting, weakness, etc. This is the reason why chemotherapy is given in limited doses, over intervals of several days, which gives the normal tissues time to recover. These are called ‘chemotherapy cycles’, as they are repeated over regular intervals. The drugs used in each cycle and the number of cycles, depends on the treatment regimen being followed, which in turn depends on the type and Stage of the cancer being treated. The side-effects of chemotherapy are nowadays very well controlled with effective drugs.
Chemotherapy is one of the foundations of modern bone sarcoma treatment and has revolutionized the management of chemo-sensitive tumors like Osteosarcoma, Ewing’s sarcoma, etc. The advances in ‘Limb Salvage Surgery’ and its establishment as the surgical treatment of choice in the management of extremity bone sarcomas is largely due to the huge improvement in survival rendered by chemotherapy. However, in some bone sarcomas like Chondrosarcoma, etc., chemotherapy has so far not been proven to be useful.
Chemotherapy is one of the foundations of modern bone sarcoma treatment and has revolutionized the management of chemo-sensitive tumors like Osteosarcoma, Ewing’s sarcoma, etc. The advances in ‘Limb Salvage Surgery’ and its establishment as the surgical treatment of choice in the management of extremity bone sarcomas is largely due to the huge improvement in survival rendered by chemotherapy. However, in some bone sarcomas like Chondrosarcoma, etc., chemotherapy has so far not been proven to be useful. Chemotherapy is a form of cancer treatment involving the use of powerful drugs, which are either injected into the body or administered through the mouth. These drugs are toxic to the cells of living tissue. Therefore, it is also called as ‘cytotoxic chemotherapy’. However, they are more toxic to cancer cells than normal cells. Hence, in the doses which are given to the patient, they kill cancer cells in much larger numbers as compared to normal cells. Nevertheless, chemotherapy does cause significant side effects like hair loss, nausea, vomiting, weakness, etc. This is the reason why chemotherapy is given in limited doses, over intervals of several days, which gives the normal tissues time to recover. These are called ‘chemotherapy cycles’, as they are repeated over regular intervals. The drugs used in each cycle and the number of cycles, depends on the treatment regimen being followed, which in turn depends on the type and Stage of the cancer being treated. A lot of the side-effects of chemotherapy are nowadays very well controlled with effective drugs. Also, the hair generally grows back within weeks of stopping the chemotherapy.
Whenever chemotherapy is employed in the treatment of a bone sarcoma, where the intention of treatment is to ‘Cure’ the patient, it is never the only treatment. Generally, the treatment begins with chemotherapy. This is called as neoadjuvant chemotherapy (chemotherapy given before the local management of a cancer). After a few cycles of neoadjuvant chemotherapy (the number of cycles depends on the chemotherapy regimen being used), the diseased area is surgically removed with wide margins (Wide Excision), and reconstructed appropriately (whenever indicated). A few days after the surgery, chemotherapy is resumed again and is continued until the entire regimen is completed, which may involve several more cycles of chemotherapy. This is called as adjuvant chemotherapy (chemotherapy given after the local management of a cancer). In some rare situations where surgery is impossible, or is very risky, and the sarcoma is sensitive to radiotherapy, instead of surgery, the patient may be treated with definitive radiotherapy along with the chemotherapy. This is especially true in the treatment of Ewing’s sarcoma affecting the spine, sacrum, etc. In some cases of bone sarcoma, radiotherapy may be used in addition to the chemotherapy and surgery.
The advantage of neoadjuvant chemotherapy is that it shrinks the tumor, which becomes smaller in size and bulk. It also reduces the vascularity of the mass, i.e. it reduces the amount of blood circulating through the mass at any given time. This reduces the blood loss during surgery. All of this makes surgery simpler and safer. Another advantage of neoadjuvant chemotherapy is that, it allows a direct way of assessing the efficacy of the neoadjuvant chemotherapy treatment. The entire tumor specimen removed during the surgery is sent to the pathologist for assessment of the surgical margins and the grading of the response of the tumor tissue to the neoadjuvant chemotherapy
Grading of response of the tumor tissue to the neoadjuvant chemotherapy is a useful method of assessing the efficacy of the chemotherapy given to the patient. Using established methods, the pathologist makes an assessment of the amount of dead (necrotic) and live (viable) tumor tissue in the entire specimen removed during the definitive surgery. By determining the relative amount of the dead tumor tissue and live tumor tissue in the specimen, the pathologist allots a grade of chemotherapy response to the particular case. An excellent response to chemotherapy indicates a good prognosis for survival.
Grading of the response to neoadjuvant chemotherapy is a useful method of assessing the efficacy of the chemotherapy given to the patient. By using established methods, the pathologist grades the response of the tumor to the neoadjuvant chemotherapy. This is done by measuring the extent of ‘tumor necrosis’ in the specimen sent to the pathologist after the definitive surgery. ‘Necrosis’, in brief, means unnatural or pathological death of tissue; which here means the unnatural death of the tumor tissue caused by chemotherapy. Chemotherapy kills tumor cells and this can be observed by the pathologist in the form of ‘tumor tissue necrosis’. The pathologist makes an assessment of the amount of dead (necrotic) and live (viable) tumor tissue in the entire specimen removed during the definitive surgery. By determining the relative amount of dead tumor tissue and live tumor tissue in the specimen, the pathologist allots a grade of chemotherapy response to the particular case. The ‘response to chemotherapy’ is considered to be best when there is 100% necrosis of the tumor tissue, i.e. there is no viable (live) sarcoma cell in the entire tumor specimen. There are several systems of ‘grading of the tumor response to chemotherapy’, developed at different centres. For example, the Picci histologic grading system of ‘response to neoadjuvant chemotherapy’, for Osteosarcoma, is as follows:
| Total Response | 100% tumor necrosis (i.e. No viable tumor cells seen) |
|---|---|
| Good Response | 90%-99% tumor necrosis (i.e. 1%-10% viable tumor cells seen) |
| Fair Response | 60%-89% tumor necrosis (i.e. 11%-40% viable tumor cells seen) |
| Poor Response | Less than 60% tumor necrosis (i.e. More than 40% viable tumor cells seen) |
An excellent response to chemotherapy indicates a good prognosis for survival. In situations with poor necrosis, this information may help in the decision of changing the chemotherapy drugs/regimen (where such an option is available). It is easy to understand why this kind of evaluation is only valid in cases where neoadjuvant chemotherapy has been used. This is generally applicable to patients with Osteosarcoma and Ewing’s sarcoma of bone.
Radiotherapy is another treatment modality, which is very useful in cancer treatment. Radiotherapy uses a certain type of energy called as ionizing radiation (which is made of powerful electromagnetic rays or high energy sub-atomic particles) to kill cancer cells and shrink tumors. Radiation therapy injures or destroys cells in the area being treated by damaging their genetic material (DNA), either killing them or affecting their ability to grow and multiply.
Radiotherapy is another treatment modality, which is very useful in cancer treatment. Radiotherapy uses a certain type of energy called as ionizing radiation (which is made of electromagnetic rays - powerful X-rays/gamma rays, or high energy sub-atomic particles like atomic nuclei, protons, neutrons, etc.) to kill/damage cancer cells and shrink tumors. Radiation therapy injures or destroys cells in the area being treated by damaging their genetic material (DNA), either killing them or affecting their ability to grow and multiply. Although radiation damages both cancer cells and normal cells, cancer cells are much more vulnerable to the damaging effects of radiation, while most normal cells can recover from its effects and function properly. This is one of the reasons why radiation in most cases is given in divided doses over a number of days, which gives normal cells/tissues time to repair and recover. This is called as ‘fractionation’ of the radiation dose. The goal of radiation therapy is to damage or destroy as many cancer cells as possible, while limiting harm to surrounding healthy tissue. When radiation is given after surgery, it is called as ‘adjuvant radiotherapy’. This is given to the region of surgery with the intention of killing any stray cancer cell or cells, which may have been inadvertently left behind, thus providing additional security against the possibility of local recurrence of the disease. Among the major bone sarcomas, radiotherapy plays an important role in the local treatment of certain cases of Ewing’s sarcoma.
Surgery has a very important role in the management of bone sarcomas. Without surgery, most of these diseases would start growing again, soon after the radiotherapy and/or chemotherapy treatment is over. In fact, in some types of bone sarcomas, surgical Wide Excision is the only way to treat the disease. The goal of surgery is to remove the cancer affected bone in such a way that the cancer does not come back again in the operated area. ‘Limb Salvage Surgery’ has established itself as the standard of care in the management of extremity bone sarcomas.
Surgery has a very important role in the management of bone sarcomas. Chemotherapy and radiotherapy, either alone or together, is not enough to treat primary bone cancers (except in certain specific situations). Without surgery, most of these diseases would start growing again, soon after the radiotherapy and/or chemotherapy treatment is over. In fact, in some types of bone sarcomas, surgical Wide Excision is the only way to treat the disease.
The goal of surgery is to remove the cancer affected bone in such a way that the cancer does not come back again in the operated area. Until a few years ago, this actually meant an amputation (surgical removal of the affected limb at an appropriate level) in most of these cases. However, in the past several years, this scenario has changed radically as Limb Salvage Surgery has established itself as the standard of care in the management of extremity bone sarcomas in our country. The problem with surgery for bone sarcomas is that, the removal of the bone tumor creates a defect in the operated bone, which needs to be repaired or reconstructed, to restore in the best possible way, the structural continuity, strength and the function of the skeletal system. Furthermore, a majority of the primary bone tumors are found close to a joint, which means that the joint function is also lost with the removal of the tumor. Very obviously, the role of surgery in the management of bone sarcomas does not end with just the proper removal of the tumor, but also extends to the restoration of the integrity of the affected bone and joint. The availability of, advanced diagnostic and imaging modalities like MRI, effective chemotherapy, radiotherapy, good quality affordable tumor prosthesis (metallic devices to replace surgically removed tumor affected bones and joints), allografts (donated human bone from a tissue bank), advanced surgical techniques and a multi-disciplinary surgical team, etc. has now made it possible to save the limb in almost 80-85% of these patients, without compromising the overall cancer treatment.
Limb salvage surgery’ means saving the limb while effectively treating the malignant bone tumor affecting it. Until some decades ago, amputation was the only treatment option for patients with extremity bone sarcomas. However, following major advances in several medical fields in the 70s and early 80s, Limb Salvage Surgery became established as the standard of care for these patients. The availability of affordable, high quality, indigenous megaprosthesis has brought Limb Salvage Surgery within the reach of most patients. In many situations, Limb Salvage Surgery is also possible without using megaprosthesis.
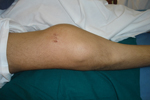
‘Limb salvage surgery’ means saving the limb while effectively treating the malignant bone tumor affecting it. Until about 35 to 40 years ago, the only treatment for a primary bone cancer in the extremities (limbs) was an amputation. Even after such a radical treatment the survival of these patients was poor; only about 20 to 25 % of these patients survived at five years from the time of treatment. Limb salvage surgery in these patients was an unrewarding experience due to several factors, chief among which were:
- Survival of these patients was very poor. Limb salvage surgery did not offer any survival benefits; in-fact it actually increased their risk of disease recurrence (for reasons mentioned in the next point), which in turn lowered the already dismal survival of these patients
- Surgery lacked precise planning as cross-sectional imaging technology (like MRI and CT scan) was not available. There was always a high risk of leaving behind disease tissue, or contamination of surgical field with tumor cells during the surgery. This resulted in a high risk of local recurrence of disease following Limb Salvage Surgery. Local recurrence of disease in sarcomas (even today) is clearly associated with a much higher risk of distant relapse (metastases) and therefore, a poor prognosis.
- Reliable ‘joint replacement’ technology was still in early stages of development.
In the 1970s and early 1980s, simultaneous breakthroughs in multiple areas of medical research and development brought about a revolution in the management of extremity bone sarcomas and firmly established Limb Salvage Surgery as the standard of care in the treatment of these enigmatic tumors.
Some of these important developments were:
- Medical oncology: At this time, the powerful role of chemotherapy in the management of some of the common bone sarcomas became clear. It brought about a dramatic improvement in the survival of these patients from 20-25% to 60-80%.
- Imaging technology: MRI and CT scan, which give excellent sectional views of the body (on which the planning of the Limb Salvage Surgery depends heavily), became available. With this technology, the margins of surgery could be planned with a high degree of accuracy and therefore limb salvage surgeries became reliable and safe.
- Joint replacement technology: Joint replacement implants and surgical techniques, revolutionized the treatment of some of the major disorders of the hip and knee joints. This same technology was extended to address the issue of managing the loss of bone and joint following Limb Salvage Surgery for bone sarcomas. To replace the surgically removed bone and joints, high quality implants and artificial joints were developed using special metals, alloys, etc.
Nevertheless, the situation here has changed in the past few years. Now, with indigenous technology, affordable, high quality and durable tumor endoprosthesis (metallic medical devices used as replacements for bone and joint) including total knee replacement implants have been developed and made available, offering excellent skeletal reconstruction options following these
surgical procedures for malignant bone tumors, providing the patient with a good functional outcome, despite the loss of large segments of bone and joint. These have been used in a large number of patients with excellent results.
Although megaprosthesis is the backbone of Limb Salvage Surgery, it does not mean Limb Salvage Surgery is not possible without the use of these implants. There are situations where only a Wide Excision of the diseased bone is sufficient. This is true when the disease affects relatively unimportant parts of some bones (which are not absolutely vital for normal function), e.g. the shaft of the fibula, the distal part of the ulna, etc. Other reconstruction options are also available, following wide excision of bone and bone segments, to restore useful function in the affected extremity. These include the use of autograft bone (bone graft harvested from the patient’s body), vascularized fibula autograft, allograft (donated human bone from a tissue bank), bone graft substitutes, local bone transports, Ilizarov technique, ECRT, etc. These are called as biological reconstruction options. It is important to know that generally, whenever a feasible biological reconstruction option is available, it is preferred over the use of metallic tumor endoprosthesis, as in the long run they provide more durable results.
In the surgery for a bone sarcoma, there are two very important steps:
- Tumor removal (Wide Excision) and
- Reconstruction of the skeletal system to restore the structural continuity, strength and function of the affected bone and/or joint.
Unlike surgery for a Soft Tissue Sarcoma, where generally the procedure ends with the Wide Excision of the tumor, in the surgery for a bone sarcoma, there are two very important steps:
- Tumor removal (Wide Excision) and
- Reconstruction of the skeletal system to restore the structural continuity, strength and function of the affected bone and/or joint.
In the first step of Limb Salvage Surgery, the bone sarcoma is always surgically removed with an appropriate margin of normal tissues, along with the biopsy scar and biopsy tract; the whole thing is removed as one single piece of tissue (en bloc). This is called as ‘Wide Excision’ of the tumor. ‘Wide Excision’ means surgical removal of the tumor tissue (with the scar and track of biopsy), along with a good margin of surrounding healthy normal tissue, in one single piece. This is done with the intention of ensuring the complete removal of the cancer tissue. Cancer begins with just one abnormal cell that eventually forms the tumor, which is made of billions of copies of this original parent cell. Therefore, hypothetically speaking, just leaving one cancer cell behind is enough for this tumor to grow back again. Thus, in the surgery for a primary malignant bone tumor (sarcoma), there is no justification for removing the tumor in pieces, as doing so defeats the very purpose of the surgery. (Even when the surgery is done with a palliative intention, the tumor is removed with a wide margin, unless such a surgery is likely to cause more morbidity, disability, and discomfort to that patient.). When important structures like blood vessels, nerves, skin, etc. are in close proximity to the tumor, the operating surgeon has to take crucial decisions on whether to preserve them or sacrifice them, based on various details like, the nature of the tumor, its grade, size, location, etc. These decisions are usually taken before surgery, based on the clinical findings, MRI images, pathology findings, etc., and appropriate plans are made to repair or reconstruct the damaged/sacrificed vital structure/s. This may therefore sometimes involve the role of other specialists, like plastic surgeons, vascular surgeons, etc., in the surgery. In some situations, where such repair or reconstruction options are not available/possible/feasible, amputation of the limb may be considered.
In the second step of Limb Salvage Surgery, appropriate reconstruction of the skeletal system is done using either biological reconstruction techniques or tumor endoprosthesis. Whenever feasible, biological reconstruction options are preferred over the use of tumor endoprosthesis. In some situations, as described earlier, reconstruction may not be necessary. However, such situations are relatively uncommon in bone sarcomas as those bones are not the usual locations for these diseases.
‘Despite receiving the best of care and completing the treatment successfully, there still remains a possibility of local recurrence of disease in the operated area and/or metastatic disease in other parts of the body, at some time in the future. This risk of relapse of bone sarcoma is highest in the first 2 to 3 years following the treatment. After this duration, the risk falls considerably.
With sarcoma, it is impossible to say with reasonable certainty that the disease will not come back, until generally at least five years from the last treatment, and that too, only when there has been no disease related problem in that interval. Despite receiving the best of care and completing the treatment successfully, there still remains a possibility of local recurrence of disease in the operated area and/or metastatic disease in other parts of the body, at some time in the future. The benefit of optimal treatment is that it reduces this risk of relapse of the disease to a large extent; however, this risk still remains significant. It is therefore, important to keep a regular follow-up with your doctor as per his/her advice and undergo regular clinical and radiological examinations as advised. Recurrence of the disease, if any, if detected in time, may be treatable. This risk of relapse of bone sarcoma is highest in the first 2 to 3 years following the treatment. After this duration, the risk falls considerably.
Relapse of sarcoma cannot be prevented with 100% certainty. However, by following the proper method for diagnosing, staging and treating the tumor, the risk of recurrence of the disease can be reduced to a large extent.
Relapse of sarcoma cannot be prevented with 100% certainty as there are several factors that contribute to this risk of recurrence, which cannot be controlled. However, by following the proper method for diagnosing, staging and treating the tumor, the risk of recurrence of the disease can be reduced to a large extent. Excellent local treatment of a non-metastatic malignant tumor, in addition to reducing the risk of local recurrence, also lowers the risk of developing metastasis. In bone sarcomas, this is usually achieved with surgery and chemotherapy, and in some cases with the additional support of radiotherapy. Even in metastatic bone sarcomas, especially those with only lung metastasis, aggressive systemic and local treatment can lower the risk of relapse.
A successful completion of treatment for cancer does not mean that the patient is cured of the disease. The patient is said to be in remission. It just means there is no detectable cancer in the body. In a significant number of patients, cancer can come back. It is important to detect any recurrence of the disease early. The importance of follow-up is that timely treatment of recurrent disease, when indicated, can improve survival.
A successful completion of treatment for cancer does not mean that the patient is cured of the disease. The patient is said to be in remission, which means that there is no sign or symptom of cancer. It does not necessarily mean there is no cancer in the body. It just means there is no detectable cancer in the body. In a significant number of patients, cancer can come back (this is called as a ‘relapse’ of the cancer). It is important to detect any recurrence of the disease early. Following successful completion of treatment for any sarcoma, patients are expected to visit the treating doctor (even when they are feeling fine and have no complaints) at regular intervals, during which they undergo a thorough clinical examination and certain investigations to look for any evidence of local, regional or distant recurrence of the disease. These investigations could be blood tests, X-ray, CT scan, MRI, sonogram, bone scan, PET scan, etc. These investigations are not done at every visit to the doctor. Generally, in bone sarcomas, follow-up is advised every three months for the first two years, followed by every six months until five years (next three years), and then yearly until ten years (next five years). The importance of follow-up is that timely treatment of recurrent disease, when indicated, can improve survival.
The following is a list of some of the primary malignant tumors of bone:
- Adamantinoma of long bones
- Chondrosarcoma
- Clear Cell Chondrosarcoma
- Hemangiopericytoma of bone
- Intraosseous Well-differentiated Osteosarcoma
- Leiomyosarcoma of bone
- Lymphoma of bone
- Mesenchymal Chondrosarcoma
- Multiple Myeloma
- Osteosarcoma
- Periosteal chondrosarcoma
- Plasmacytoma
- Post – radiation Sarcoma
- Synovial Chondrosarcoma
- Ewing’s sarcoma of bone, Primitive Neuroectodermal Tumor (PNET) of bone
- Angiosarcoma
- Chordoma
- Fibrosarcoma
- High-Grade Surface Osteosarcoma
- Juxtacortical Chondrosarcoma
- Liposarcoma of bone
- Malignant Fibrous Hystiocytoma (MFH)
- Multifocal Osteosarcoma
- Non-Hodgkin’s Lymphoma of bone
- Parosteal Osteosarcoma
- Periosteal Osteosarcoma
- Post - Paget’s Sarcoma
- Small Cell Osteosarcoma
- Telangiectatic Osteosarcoma

